The Endosymbiont Hypothesis: Things Aren’t What They Seem to Be
Sometimes, things just aren’t what they seem to be. For example, when it comes to the world of biology:
- Fireflies are not flies; they are beetles
- Prairie dogs are not dogs; they are rodents
- Horned toads are not toads; they are lizards
- Douglas firs are not firs; they are pines
- Silkworms are not worms; they are caterpillars
- Peanuts are not nuts; they are legumes
- Koala bears are not bears; they are marsupials
- Guinea pigs are not from Guinea and they are not pigs; they are rodents from South America
- Banana trees are not trees; they are herbs
- Cucumbers are not vegetables; they are fruit
- Mexican jumping beans are not beans; they are seeds with a larva inside
And . . . mitochondria are not alphaproteobacteria. In fact, evolutionary biologists don’t know what they are—at least, if recent work by researchers from Uppsala University in Sweden is to be taken seriously.1
As silly as this list may be, evolutionary biologists are not amused by this latest insight about the identity of mitochondria. Uncertainty about the evolutionary origin of mitochondria removes from the table one of the most compelling pieces of evidence for the endosymbiont hypothesis.
A cornerstone idea within the modern evolutionary framework, biology textbooks often present the endosymbiont hypothesis as a well-evidenced, well-established evolutionary explanation for the origin of complex cells (eukaryotic cells). Yet, confusion and uncertainty surround this idea, as this latest discovery attests. To put it another way: when it comes to the evolutionary explanation for the origin of complex cells in biology textbooks, things aren’t what they seem.
Most evolutionary biologists believe that the endosymbiont hypothesis is the best explanation for one of the key transitions in life’s history—namely, the origin of complex cells from bacteria and archaea. Building on the ideas of Russian botanist Konstantin Mereschkowski, Lynn Margulis (1938–2011) advanced the endosymbiont hypothesis to explain the origin of eukaryotic cells in the 1960s.
Since that time, Margulis’s ideas on the origin of complex cells have become an integral part of the evolutionary paradigm. Many life scientists find the evidence for this hypothesis compelling; consequently, they view it as providing broad support for an evolutionary explanation for the history and design of life.
According to this hypothesis, complex cells originated when symbiotic relationships formed among single-celled microbes after free-living bacterial and/or archaeal cells were engulfed by a “host” microbe. (Ingested cells that take up permanent residence within other cells are referred to as endosymbionts.)
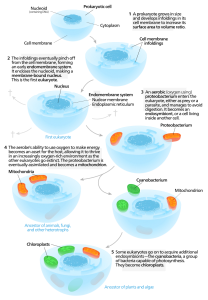
The Evolution of Eukaryotic Cells According to the Endosymbiont Hypothesis
Image source: Wikipedia
Presumably, organelles such as mitochondria were once endosymbionts. Evolutionary biologists believe that once taken inside the host cell, the endosymbionts took up permanent residence, with the endosymbiont growing and dividing inside the host. Over time, endosymbionts and hosts became mutually interdependent, with the endosymbionts providing a metabolic benefit for the host cell. The endosymbionts gradually evolved into organelles through a process referred to as genome reduction. This reduction resulted when genes from endosymbionts’ genomes were transferred into the genome of the host organism. Eventually, the host cell evolved machinery to produce proteins needed by the former endosymbiont and processes to transport those proteins into the organelle’s interior.
Evidence for the Endosymbiont Hypothesis
The morphological similarity between organelles and bacteria serve as one line of evidence for the endosymbiont hypothesis. For example, mitochondria are about the same size and shape as a typical bacterium and they have a double membrane structure like the gram-negative cells. These organelles also divide in a way that is reminiscent of bacterial cells.
Biochemical evidence also seems to support the endosymbiont hypothesis. Evolutionary biologists view the presence of the diminutive mitochondrial genome as a vestige of this organelle’s evolutionary history. Additionally, biologists also take the biochemical similarities between mitochondrial and bacterial genomes as further evidence for the evolutionary origin of these organelles.
The presence of the unique lipid cardiolipin in the mitochondrial inner membrane also serves as evidence for the endosymbiont hypothesis. Cardiolipin is an important lipid component of bacterial inner membranes. Yet, it is not found in the membranes of eukaryotic cells—except for the inner membranes of mitochondria. In fact, biochemists consider it a signature lipid for mitochondria and a vestige of this organelle’s evolutionary history.
But, as compelling as these observations may be, for many evolutionary biologists phylogenetic analysis provides the most convincing evidence for the endosymbiont hypothesis. Evolutionary trees built from the DNA sequences of mitochondria, bacteria, and archaea place these organelles among a group of microbes called alphaproteobacteria. And, for many (but not all) evolutionary trees, mitochondria cluster with the bacteria, Rickettsiales. For evolutionary biologists, these results mean that the endosymbionts that eventually became the first mitochondria were alphaproteobacteria. If mitochondria were not evolutionarily derived from alphaproteobacteria, why would the DNA sequences of these organelles group with these bacteria in evolutionary trees?
But . . . Mitochondria Are Not Alphaproteobacteria
Even though evolutionary biologists seem certain about the phylogenetic positioning of mitochondria among the alphaproteobacteria, there has been an ongoing dispute as to the precise positioning of mitochondria in evolutionary trees, specifically whether or not mitochondria group with Rickettsiales. Looking to bring an end to this dispute, the Uppsula University research team developed a more comprehensive data set to build their evolutionary trees, with the hope that they could more precisely locate mitochondria among alphaproteobacteria. The researchers point out that the alphaproteobacterial genomes used to construct evolutionary trees stem from microbes found in clinical and agricultural settings, which is a small sampling of the alphaproteobacteria found in nature. Researchers knew this was a limitation, but, up to this point, this was the only DNA sequence data available to them.
To avoid the bias that arises from this limited data set, the researchers screened databases of DNA sequences collected from the Pacific and Atlantic Oceans for undiscovered alphaproteobacteria. They uncovered twelve new groups of alphaproteobacteria. In turn, they included these new genome sequences along with DNA sequences from previously known alphaproteobacterial genomes to build a new set of evolutionary trees. To their surprise, their analysis indicates that mitochondria are not alphaproteobacteria.
Instead, it looks like mitochondria belong to a side branch that separated from the evolutionary tree before alphaproteobacteria emerged. Adding to their surprise, the research team was unable to identify any bacterial species alive today that would group with mitochondria.
To put it another way: the latest study indicates that evolutionary biologists have no candidate for the evolutionary ancestor of mitochondria.
Does the Endosymbiont Hypothesis Successfully Account for the Origin of Mitochondria?
Evolutionary biologists suggest that there’s compelling evidence for the endosymbiont hypothesis. But when researchers attempt to delineate the details of this presumed evolutionary transition, such as the identity of the original endosymbiont, it becomes readily apparent that biologists lack a genuine explanation for the origin of mitochondria and, in a broader context, the origin of eukaryotic cells.
As I have written previously, the problems with the endosymbiont hypothesis are not limited to the identity of the evolutionary ancestor of mitochondria. They are far more pervasive, confounding each evolutionary step that life scientists envision to be part of the emergence of complex cells. (For more examples, see the Resources section.)
When it comes to the endosymbiont hypothesis, things are not what they seem to be. If mitochondria are not alphaproteobacteria, and if evolutionary biologists have no candidate for their evolutionary ancestor, could it be possible that they are the handiwork of the Creator?
Resources
- “Evolutionary Paradigm Lacks Explanation for Origin of Mitochondria and Eukaryotic Cells” by Fazale Rana (article)
- “Why Do Mitochondria Have DNA?” by Fazale Rana (article)
- “Mitochondrial Genomes: Evidence for Evolution or Creation?” by Fazale Rana (article)
- “Can a Creation Model Explain the Origin of Mitochondria?” by Fazale Rana (article)
- “Complex Protein Biogenesis Hints at Intelligent Design” by Fazale Rana (article)
- “Mitochondria’s Deviant Genetic Code: Evolution or Creation?” by Fazale Rana (article)
- “Biology’s Big Bangs” by Fazale Rana (article)
Endnotes
- Joran Martijn et al., “Deep Mitochondrial Origin Outside the Sampled Alphaproteobacteria,” Nature 557 (May 3, 2018): 101–5, doi:10.1038/s41586-018-0059-5.





