Water: Designed for Life, Part 2 (of 7)
Guest writers John Millam, Ken Klos, and Iain D. Sommerville continue their exploration of water with a look at how intermolecular forces influence water’s boiling and melting points.
“Ocean, n. A body of water occupying about two-thirds of a world made for man—who has no gills.” Ambrose Bierce, The Devil’s Dictionary
It’s easy to take Earth’s oceans for granted. These large bodies of water are a familiar sight—covering more than 70 percent of the planet’s surface. The mere presence of liquid water (oceans, lakes, and rivers) plays a critical role in supporting life. Given the rarity of even trace amounts of liquid water in our galaxy, its abundance on Earth reflects amazing fine-tuning of our planet and solar system (a topic we will address later in this series). Today, we will examine the more-immediate question of why it is possible for water to be a liquid (rather than a gas) at ambient temperatures.
Intermolecular Forces
In part 1 of this series, we examined water’s molecular structure. Each water molecule consists of two hydrogens covalently bonded (via two pairs of shared electrons) to a central oxygen atom. These covalent bonds are extremely strong and hold the molecule together. Here in part 2, we will switch from considering intramolecular forces(bonding between atoms in the same molecule) to intermolecular forces (attractions between different molecules).
Consider the countless numbers of individual water molecules in a glass of water. These molecules are bound together by a web of attractive intermolecular forces to form a liquid. The nature and strength of these forces determine the macroscopic properties of water, such as its boiling point.
For example, if water’s intermolecular forces were considerably weaker, then water would be a gas (except at extremely low temperatures) because the thermal motion pushing the molecules apart would be greater than the intermolecular forces holding them together. Only when the intermolecular forces are strong enough, can they overcome the thermal motion (below a certain temperature) and allow the substance to condense into a liquid. This tipping point between behaving as a gas and a liquid is the boiling point. The stronger the intermolecular forces, the higher the boiling point will be. If they are strong enough (as in the case of water) then the boiling point will be high enough for the substance to exist as a liquid at normal temperatures (i.e., what we experience on our planet’s surface).
Of course, if the intermolecular forces are too strong (at a given temperature) the molecules will bind together to form a solid rather than a liquid. This corresponds to the melting point. In order for much of the earth to be covered in liquid water, the intermolecular forces must be strong (much stronger than would be expected for a small molecule)—yet not too strong.
Hydrogen Bonding
There are several types of intermolecular forces—but only hydrogen bonding is critical to our current discussion. Despite its name, a “hydrogen bond” is not a true chemical bond (since no electrons are shared). Rather, it is a special type of electrostatic attraction. A hydrogen bond is analogous to two magnets sticking together because their opposite poles attract. Hydrogen bonds function as a strong bridge or connection between neighboring molecules so that they stick together (e.g., causing water to behave as a liquid rather than a gas at room temperature). And since hydrogen bonds are nowhere near as strong as true chemical bonds, they can easily break and reform (similar to the separation and reconnection of magnets).
Two noteworthy characteristics of hydrogen bonds are worth mentioning here. First, they are strong compared to the other intermolecular forces for small molecules. Thus, they play a major role in determining water’s properties.1 Second, unlike the other intermolecular forces, hydrogen bonds form only when certain conditions are met. Consequently, substances that contain hydrogen bonds display significantly different behavior and properties when compared to similar molecules that lack these bonds.
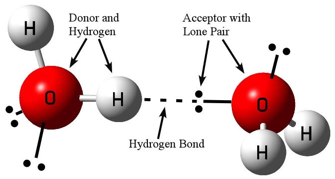
Forming hydrogen bonds requires one atom to act as a hydrogen “donor” and an adjacent atom to act as an “acceptor.”2 The donor atom must be a highly electronegative atom bonded to a hydrogen atom.3 Of all the elements, only nitrogen (N), oxygen (O), and fluorine (F) are sufficiently electronegative to permit significant hydrogen bonding. The acceptor atom must likewise be highly electronegative (again primarily N, O, or F) and possess a “lone pair” of electrons (two electrons that are not involved in covalent bonding). The donor causes its hydrogen atom to have a partial positive charge, which is then attracted to the negatively charged lone pair on the acceptor. Lastly, the donor, its hydrogen, the acceptor, and its lone pair must all be arranged along a nearly straight line (see figure 1).4
Figure 1: Diagram of hydrogen bonding between water molecules
Image credit: John Millam
Importance of Hydrogen Bonding
To understand how important hydrogen bonding is, it is helpful to compare the boiling point of water to those of closely related compounds. Water is a hydride of oxygen—an element bonded to an appropriate number of hydrogen atoms (two hydrogens in the case of oxygen). Therefore, we will compare it to hydrides of elements located close to oxygen in the periodic table. Figure 2 shows the boiling points of these hydrides, with lines connecting elements of the same “family” (belonging to the same column of the periodic table).5
If we ignore for a moment three anomalous boiling points—those of water (H2O), ammonia (NH3), and hydrogen fluoride (HF)—we see a clear trend of boiling points increasing toward the right. This is the result of another intermolecular force (called London dispersion), which increases in strength as the number of electrons increases. From this, we can extrapolate what the approximate boiling points of the anomalous three—H2O, NH3, and HF—would be if hydrogen bonding were not present (see red dashed lines in figure 2).
If water didn’t possess hydrogen bonding, we would expect it to reach the boiling point around -100 °C (-148 °F)—rather than 100 °C (212 °F). This is an enormous difference! The equivalent graph for melting points (not shown) follows a similar trend. Clearly, without hydrogen bonding, water would be a gas at normal temperatures and so could not serve as a medium for cellular processes. Neither would there be rivers, lakes, or oceans—not to mention people!
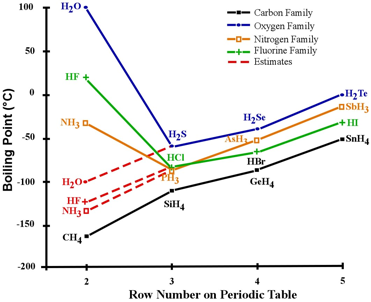
Figure 2: Boiling points of certain hydrides.
The red dashed lines approximate the boiling points of H2O, NH3, and HF in the absence of hydrogen bonding.
Image credit: John Millam
Comparison of Water, Ammonia, and Hydrogen Fluoride
The boiling points of water, ammonia, and hydrogen fluoride are clearly exceptional when compared to those of other similar hydrides. But water’s high boiling point stands head and shoulders above these two nearest competitors in exceptionality. This difference merits a more detailed look.
Fluorine is more electronegative than oxygen; so HF’s hydrogen bonds are the strongest of the three—yet water has the highest boiling point. This is because water can form up to four hydrogen bonds per molecule (see figure 3) whereas the others are limited to just two. Thus, water molecules can form a hydrogen-bonded network that extends in all directions. Any force applied to one water molecule will be transmitted to all of the other molecules via these hydrogen bonds. In contrast, ammonia and hydrogen fluoride can form only small hydrogen-bonded clusters.
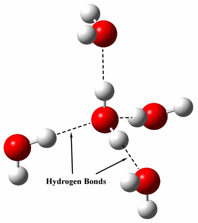
Figure 3. Four water molecules oriented, as required by their hydrogen bonds, around a fifth central molecule
Each of the four surrounding molecules can hydrogen bond to three other water molecules, resulting in a continuous web of hydrogen bonds throughout the entire body of water.
Image credit: John Millam
Water is able to form four hydrogen bonds because it possesses two hydrogens (donors) and two lone pairs of electrons on the oxygen (acceptors). HF has three lone pairs (donors) but only one hydrogen atom (acceptor), so as a pure liquid each HF molecule would typically participate in only two hydrogen bonds.6 NH3 has the opposite problem, having three donors but only one lone pair. As with HF, pure NH3 can participate in only two hydrogen bonds per molecule.7
Conclusion
From a chemist’s point of view, water is absolutely astonishing. Given its simple composition, we would expect it to be a gas at ambient temperatures, yet it is a liquid. In fact, it is the only naturally occurring, inorganic liquid found in abundance.8 Moreover, we owe our life to this fact because without liquid water life would be impossible. In our opinion, there is no doubt that the Creator designed the water molecule to fulfill its unique and crucial roles in supporting life.
In the remaining parts of this study, we will point out still more evidences from water for the Creator’s role in our existence
To access additional parts, please click on a link below:
Part 1 | Part 2 | Part 3 | Part 4 | Part 5 | Part 6 | Part 7

Dr. John Millam received his PhD in theoretical chemistry from Rice University in 1997, and currently serves as a programmer for Semichem in Kansas City.

Mr. Ken Klos received his MS in environmental studies from the University of Florida in 1971, and worked as an environmental/civil engineer for the state of Florida.
Iain D. Sommerville
Endnotes
- Hydrogen bonds in water are about 5 kcal/mol in energy, whereas van der Waals force (another intermolecular force) in water is only a feeble 0.3 kcal/mol. For perspective, water’s covalent bonds are about 100 kcal/mol each. John D. Barrow and Frank J. Tipler, The Anthropic Cosmological Principle (New York: Oxford University Press, 1984), 526.
- Typically, the donor and acceptor are located on different molecules, but hydrogen bonding can also occur between different atoms in the same molecule. An important example of this is hydrogen bonding between different amino acids in a protein. In this case, these hydrogen-bonded amino acids are responsible for the secondary structure of the molecule.
- One additional caveat: the donor atom must also be small. Only elements in the first row of the periodic table (notably N, O, and F) satisfy this criterion. So, while chlorine (Cl) is just as electronegative as N, its larger radius precludes it from having significant hydrogen bonding.
- Frank H. Stillinger, “Water Revisited,” Science 209 (July 25, 1980): 451–52.
- A “family” or column of the periodic table represents elements with very similar chemical properties. For example, the carbon family contains carbon (C), silicon (Si), germanium (Ge), and tin (stannum or Sn). The members of this family all naturally bond to four hydrogen atoms. In contrast, the nitrogen family bonds to three hydrogens, the oxygen family to two, and the fluoride family to only one.
- This refers to HF’s average behavior as a pure liquid. Individual HF molecules could form up to four hydrogen bonds. Nevertheless, the overall the number of hydrogen bonds possible is limited by the fact that HF has just one hydrogen atom per molecule to act as donor.
- As with HF, this describes the average behavior of pure liquid NH3 where, in this case, hydrogen bonding is limited by the fact that each molecule possesses only one lone pair.
- J. C. Bailer Jr. et al., Chemistry, 2nd ed., (Orlando, FL: Academic Press, 1984), 425.