Highly Fine-Tuned Reverse Weathering Stabilized Earth’s Early Climate
It bothers my wife Kathy that I can be sitting in front of a computer and not even notice that the room temperature has dropped or risen by more than ten degrees Fahrenheit. Unlike me, Kathy is extremely sensitive to temperature changes. A temperature change in our home or in one of our offices as small as a half degree is enough for her to declare that she is too cold or too warm. Her temperature sensitivity is even better than any of our thermostats.
Faint Young Sun
Now, two Yale University geologists, Terry Isson and Noah Planavsky, have discovered that Earth also possessed an amazing thermostat that stabilized its climate and ocean pH (measure of acidity or basicity of an aqueous solution) from 3.5–0.54 billion years ago.1 Such a highly fine-tuned thermostat is necessary for life to be sustained on Earth for a few billion years. The reason why is that the Sun gradually brightens as it continues to fuse hydrogen into helium in its nuclear furnace.
As I explain in my book Improbable Planet,2 since the origin of life 3.825 billion years ago, the Sun has become 18–23 percent brighter (see figure 1). In chapter 12 I describe how fine-tuned timing and degree of weathering of continental silicates and burial of organic carbon from 543 million years ago to the present continuously removed just the right quantities of greenhouse gases from Earth’s atmosphere to perfectly compensate for the Sun’s ever-increasing brightness.
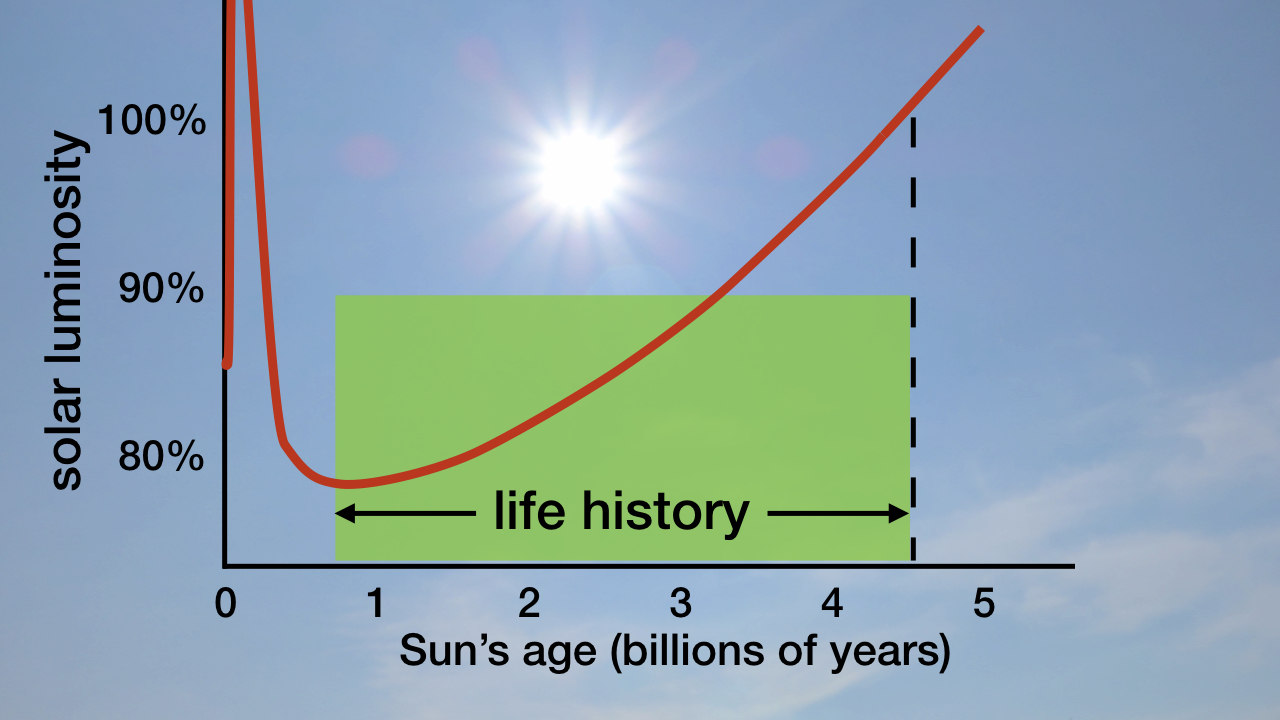
Figure 1: The Sun’s Brightness (Luminosity) throughout Its History. Image credit: Hugh Ross
However, geologists lacked a satisfactory explanation for the thermostat that regulated the temperature of Earth’s surface during life’s history previous to 543 million years ago. In particular, researchers have sought to account for the maintenance of high partial pressures of carbon dioxide in the atmosphere at levels 10–100 times the present. Such levels are essential for preventing runaway global glaciations (see figure 2) in the context of a much dimmer Sun.

Figure 2: Lack of Greenhouse Gases Produces a Global Glaciation Event. Without adequate greenhouse gases in the atmosphere, ice fields like these would cover all of Earth’s surface. Image credit: Hugh Ross
In their paper Isson and Planavsky first cite research studies3 demonstrating why previous proposals—reduction in weatherability of Earth’s crust, changes in the composition of Earth’s crust, different rates of continental uplift, different sea levels—prove inadequate for maintaining such high partial pressures of carbon dioxide. They then suggest a mechanism and develop a detailed model based on that mechanism that explains how the needed high partial carbon dioxide pressures in the atmosphere can be sustained for the three billion years previous to the Cambrian explosion.
Reverse Weathering
The authors propose a robust natural thermostat—reverse weathering. They demonstrate that the silica-rich conditions that dominated Earth’s oceans during the Precambrian eras would have created minerals rather than breaking them down.
The silicate weathering reaction refers to how rain falling on continental silicates acts as a catalyst to transform silicates into carbonates and sand while removing carbon dioxide from the atmosphere. The chemical equation below shows the specific reaction, where X is a metal element:
XSiO3 + 2CO2 +3H2O → XCO3 + SiO2 + CO2 + 3H2O
Note that for every silicate (XSiO3) molecule converted into a carbonate (XCO3) molecule and a sand molecule (SiO2), one carbon dioxide (CO2) molecule is removed from the atmosphere.
This continual removal of carbon dioxide from the atmosphere causes Earth’s atmosphere to progressively trap less of the Sun’s ever-increasing heat after the Cambrian explosion. Since the Cambrian explosion, this continual removal of carbon dioxide has perfectly compensated for the Sun’s increasing brightness, thereby maintaining an ideal temperature on Earth’s surface for abundant, diverse life.
In Isson and Planavsky’s proposed reverse weathering reaction, instead of silicates being broken down into carbonates and sand, they are built up into clays. Specifically, dissolved silica in the oceans reacts with alkali metal cations to make different kinds of nonkaolinite clays. The two chemical equations below, where X is a metal element, show the reactions that were operating in Earth’s early oceans:
3X2+ + 2H4SiO4 + 6HCO3– → X3Si2O3(OH)4 + 6CO2 + 5H2O
Al4Si4O10(OH)8 + 3Fe2+ + 6HCO3– → Fe3Al2Si4O10(OH)8 + 2Al(OH3) + 6CO2
In their paper the two geologists show eleven different reverse weathering reactions that would have operated in Earth’s early oceans to produce eleven different kinds of authigenic (formed in the locations where it is found) clay.4
For every clay molecule produced in these reactions, six carbon dioxide molecules are released into the atmosphere. This powerful injection of carbon dioxide into Earth’s early atmosphere explains why, in spite of the Sun being much dimmer, Earth’s surface temperature remained warm enough for abundant life from 3.82–0.54 billion years ago.
Fine-Tuned Rates of Reverse Weathering
Isson and Planavsky demonstrate that clay formation in the oceans “varied dramatically” from 3.8 billion years ago to the present.5 The gradual decline in clay formation was due partly to the gradual depletion of silica in the oceans as the silica reacted to form clays. Another big factor was Earth’s life. Different life-forms during different eras in Earth’s history progressively harvested more and more silica from the marine environment. While the fossil record before the Avalon explosion, 575 million years ago, is not complete enough to accurately discern the sequence of life that gradually reduced marine silica concentrations, it is complete from that time forward.
The Avalon explosion marks the time when animals first appeared on Earth. During the Cambrian explosion (543 million years ago) complex animals first appeared.
Silicifying (becoming saturated with silica) sponges first appeared shortly after the Avalon explosion and radiated throughout Earth’s oceans during the early Cambrian period (543–520 million years ago). During the Ordovician period (485–444 million years ago), radiolarians joined silicifying sponges in efficiently lowering marine silica concentrations. Later, the rapidly growing population and diversity of diatoms during the Cretaceous period (145–66 million years ago) and beyond accelerated the removal of marine silica. Meanwhile, the appearance and later multiplication and diversification of vascular plants on the continents accelerated silicate weathering.
Reverse Weathering Points to Intelligent Agency
The bottom line is that it takes a very carefully designed sequence of different life-forms at the just-right abundance and diversity levels introduced at the just-right times to ensure that the just-right amounts of carbon dioxide reside in Earth’s atmosphere so as to perfectly compensate for the changes in the Sun’s luminosity throughout the past 3.82 billion years. Nothing less than a super-intelligent, supernatural Being can explain such exquisitely fine-tuned creation activity.
A remaining question is why would the Creator expend so much time and effort in compensating for the Sun’s changing luminosity? Couldn’t such a powerful Creator just place humans on Earth at the one time in the Sun’s history when its flares wouldn’t wipe them out?
The Creator indeed could have done this without altering any of the laws of physics. However, humans would have lacked the benefit of 76+ quadrillion tonnes (1 tonne = 1.102 tons) of biodeposits. This enormous treasure chest of the remains of previous life (e.g., limestone, marble, coal, oil, and natural gas) allowed humans to quickly launch and sustain global high-technology civilization.
Featured image: Authigenic Minerals in a Marine Sediment. Image credit: Hannes Grobe
Endnotes
- Terry T. Isson and Noah J. Planavsky, “Reverse Weathering as a Long-Term Stabilizer of Marine pH and Planetary Climate,” Nature 560 (August 23, 2018): 471–75, doi:10.1038/s41586-018-0408-4.
- Hugh Ross, Improbable Planet: How Earth Became Humanity’s Home (Grand Rapids, MI: Baker, 2016), 154–57.
- Lee R. Kump, Susan L. Brantley, and Michael A. Arthur, “Chemical Weathering, Atmospheric CO2, and Climate,” Annual Reviews of Earth and Planetary Sciences 28 (2000): 611–67, doi:10.1146/annurev.earth.28.1.611; C. K. Keller and B. D. Wood, “Possibility of Chemical Weathering before the Advent of Vascular Land Plants,” Nature 364 (July 15, 1993): 223–25, doi:10.1038/364223a0.
- Isson and Planavsky, “Reverse Weathering,” 472.
- Isson and Planavsky, “Reverse Weathering,” 472.






