Water: Designed for Life, Part 7 (of 7)
Guest writers John Millam, Ken Klos, and Iain D. Sommerville wrap up this seven-part series on water with a discussion of liquid water’s rarity throughout our solar system. It seems only Earth has maintained the right amount of liquid water for the support of advanced life.
Water, water, every where,
And all the boards did shrink,
Water, water every where,
Nor any drop to drink
This well-known stanza from Samuel Taylor Coleridge’s poem “The Rime of the Ancient Mariner” expresses the strange paradox familiar to all sailors—being surrounded by an abundance of water yet unable to drink it. A human will die within just a few days without fresh, salt-free water. What a strange irony that someone could die of thirst in the middle of an ocean full of water.
As we’ve seen throughout this series’ preceding segments, water reveals astonishing design. Water is not merely exceptional—it is unique in many ways that are critical for supporting life. And water’s exceptionality doesn’t end there. The abundance of liquid water on Earth is further evidence for the design of our planet and solar system.
The Abundance of Liquid Water on Earth
Earth is uniquely and distinctively the water planet. Oceans and seas cover almost 71 percent of the planet’s surface to an average depth of 2.3 miles—that’s over 12,000 feet and deeper than most mountains are tall! If we add lakes, rivers, glaciers, and icecaps, then water covers almost 75 percent of Earth. In total, the Earth contains approximately 1.368 billion cubic kilometers (more than 332 million cubic miles) of water.1
As Coleridge’s poem demonstrates, water lies at the heart of a galactic paradox. Water is the third most abundant molecule in the universe2—yet liquid water is exceptionally rare! Earth, on the other hand, is so blessed with liquid water that we take it for granted, even waste and squander it. How do we account for this amazing abundance?
A Tale of Two Planets
In some ways, Venus and Mars are Earth’s near-identical twins (see figure 1). Venus’ diameter is only 5 percent smaller than Earth’s, while Mars’ mass is about one-tenth of Earth’s mass. Their orbits are similar to Earth’s, though Venus is 30 percent closer to the Sun and Mars 50 percent farther from it. Given these factors, one might expect these two planets to share Earth’s ability to sustain liquid water. Indeed, until just a few decades ago, many people assumed Mars was a paradise filled with intelligent life.3However, actual probes revealed that both planets are devoid of liquid water and life. Is water-rich Earth the galactic rule, and Mars and Venus the exception—or is the reverse true?
Figure 1: Size comparison of Mercury, Venus, Earth, and Mars in true color.
Image credit: Wikimedia Commons/Scooter20 based on Mercury (NASA/JPL); Venus (NASA/Image processing by R. Nunes; Earth (NASA); Mars (NASA and The Hubble Heritage Team (STScI/AURA)
Scientists now know that, in its youth, Venus contained water and experienced a more temperate climate. Today it is a dry, scorching world.4 Its crushingly-dense atmosphere has air pressures 90 times those on Earth. This thick, heat-trapping atmosphere causes temperatures on Venus’ surface to reach 480°C (900°F). So what transformed a Cinderella planet into an ugly stepsister? While scientists are still searching for a complete answer to this question, runaway overheating and loss of water due to the thick atmosphere presents one possible explanation.
Given Earth’s larger mass, its atmosphere should have been even thicker than Venus’—which would have prevented Earth from supporting water or life. However, the collision event that resulted in the Moon’s formation stripped away early Earth’s Venus-like atmosphere.5 The atmosphere that emerged afterwards is forty times thinner than what we find on Venus, thus allowing Earth to remain cool and wet.
Like Venus, Mars started with liquid water. Its surface today, however, is desiccated and frozen. Mars’ distance from the Sun and almost nonexistent atmosphere (stripped away as a result of the planet’s smaller mass and closer proximity to Jupiter) led to runaway freezing, with the average surface temperature sitting at a frigid -55°C (-67°F).6 The lack of atmosphere also means that any surface water on Mars would evaporate immediately. Some water may remain, but only as permafrost trapped beneath the surface or as ice near the poles. Liquid water may occasionally burst forth (e.g., due to volcanic heating) and create erosion patterns, but such water would not last long.7 Without any long-standing water, Mars will remain devoid of life.8
Our Solar System
The rest of the planets in our solar system are even more hostile to life. Closest to the Sun, Mercury can reach a scorching 427°C (800°F)—much too hot for liquid water. In the other direction, we have Saturn, Jupiter, Uranus, and Neptune, all far too cold. Even if they do contain water, it would be as solid ice only.
Curiously, the one other known place with liquid water in our solar system is not a planet at all, but Europa, one of Jupiter’s moons (see figure 2).9 Europa’s surface temperature is -162°C (-260°F), but tidal heating of its core (caused by Jupiter’s gravity) keeps a subsurface layer of water in a liquid state. Current estimates indicate Europa possesses a shell of ice about 10 miles thick with 50 miles of ocean beneath it.10 This data has inspired some people to speculate about the possibility of life on Europa, but, while there are significant arguments against this notion, no real evidence exists to support it.11
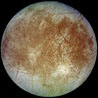
Figure 2: Jupiter’s moon Europa
Image credit: NASA/JPL/DLR
Habitable Zone
From this analysis of our solar system we see the basis for a well-known principle—planets too close to their star will be too hot for life, while those too far away will be too cold. In our solar system, only Earth lies in the “Goldilocks” region between these two hostile extremes where liquid water can remain over much of the planet’s surface. Astronomers refer to this region as the “habitable zone” because planets outside it cannot maintain liquid water and, thus, cannot support life.12
The habitable zone’s location depends primarily on the star’s brightness (or star type). For example, the cooler and dimmer the star the closer the habitable zone will reside. A variety of other factors, such as the nature and the thickness of the planet’s atmosphere, also modify the habitable zone.
However, because each star’s brightness varies over its lifetime (the Sun has brightened by 30 percent over the last 4.5 billion years) the habitable zone changes accordingly.13 For example, if the star brightens, the habitable zone will move farther out. To maintain liquid water and life, a planet must reside inside the habitable zone throughout its lifetime. The range of distances for which habitability remains feasible is called the continuously habitable zone (CHZ); consequently the CHZ is far narrower than the habitable zone in general.
Exoplanets
When the search for extrasolar planets (exoplanets) began, researchers expected to find many warm, watery Earth-like planets based on early models that suggested most planetary systems should be similar to our own.
The first positive detection of an exoplanet came in 1995; since then hundreds more have been found. Currently (these numbers increase frequently) there are 861 confirmed exoplanets in 677 planetary systems as well as 2,740 planet candidates. However, contrary to expectations, this sampling of exoplanets has failed to find any solar systems with a planet capable of hosting any life-forms. Many scientists are still optimistic that such planets will be found, but none have been found to date.14 For example, many possess Jupiter-like planets orbiting very close to their stars, a situation that would disrupt small rocky planets within the system.15 These data strongly underscore Earth’s uniqueness.
Retaining Liquid Water
Water retention is undesirable in people, but critical for a planet’s habitability. Typically, planets start with a quantity of water and may gain additional amounts from the influx of comets. This was certainly true of our neighbors Venus and Mars, yet today they are both dry and dead. So why has Earth maintained a copious supply of water?
A planet’s temperature and surface gravity determine its ability to retain water and other gases for billions of years. Together these factors decide which molecules have enough energy to escape the planet’s gravity and dissipate into space. Because a planet’s mass is the main determinant for its surface gravity, smaller planets’ surface gravity is too low to retain water (e.g., Mars and perhaps Venus) while more massive planets retain too many gases (e.g., Jupiter and Saturn).
Earth, on the other hand, demonstrates a remarkable degree of fine-tuning. Its surface gravity is high enough to preserve its water (molecular weight 18), yet low enough to allow methane (weight 16) and ammonia (weight 17) to dissipate into space. (Both of these greenhouse gases are toxic to life.) Earth’s mass and surface temperature must be just right, within a few percent, to retain our oceans and not suffocate life-forms with methane and ammonia.16
But Not Too Much Water
Surprisingly, the well-known expression “the more, the merrier” may not always apply to the amount of water a planet holds. Even given how beneficial water is, too much would be detrimental to habitability. Dry land and shoreline areas are critical niches for life, while deep oceans are comparable to deserts (in terms of biodiversity). If Earth were a water world with all the continents submerged, its capacity to support diverse life would be severely limited.17 Advanced life like human beings would certainly have been impossible.
Astronomers have discovered that such a fate was a real possibility for Earth. The solar nebula that gave birth to our solar system contained a lot of water that was incorporated into the formation of planets. If we assume Earth received the nebula’s average concentration, our planet would have had 1,000 times more water than it does now.18 Though much of that water was lost during Earth’s formation stage, it still would have retained far too much. However, the same Moon-forming collision that helped thin early Earth’s atmosphere also stripped away most of the excess water.19 That event had to be extremely fine-tuned to remove just enough water to allow for the formation of large continents while still allowing the planet to keep an abundance of the vital fluid.
Conclusion—Rare Earth
Despite water’s ubiquitous abundance throughout the galaxy, significant quantities of liquid water, retained for cosmically significant periods of time, are extremely unlikely. Specifically, the planet must:
- Orbit its parent star at the right distance (i.e., be located within the CHZ);
- Have the right atmosphere—not too thick or too thin;
- Have gravity high enough to retain liquid water yet low enough to allow other light gases to escape the atmosphere; and
- Maintain these conditions at relatively constant levels for at least several billion years.
Liquid water’s rarity demonstrates that Earth is truly an extraordinary jewel. It also poses a serious dilemma for those searching for life on other planets since liquid water is essential to supporting life. (We hope to address the possibility of life existing without water in a future set of articles.) For Christians, however, water is just one more reason to praise the Creator who designed our habitat for our benefit.
To access additional parts, please click on a link below:
Part 1 | Part 2 | Part 3 | Part 4 | Part 5 | Part 6 | Part 7

Dr. John Millam received his PhD in theoretical chemistry from Rice University in 1997, and currently serves as a programmer for Semichem in Kansas City.

Mr. Ken Klos received his MS in environmental studies from the University of Florida in 1971, and worked as an environmental/civil engineer for the state of Florida.
Dr. Iain D. Sommerville
Dr. Iain D. Sommerville received his PhD from the University of Strathclyde, Glasgow, Scotland in 1966, and currently serves as professor emeritus of materials science and engineering at the University of Toronto.
Endnotes
- U.S. Geological Survey, “How Much Water Is There On, In, and Above the Earth?”, last modified May 23, 2013, accessed April 14, 2013, https://ga.water.usgs.gov/edu/earthhowmuch.html.
- Hydrogen (H2) and protonated hydrogen (H3) are the two most common molecules in the universe.
- Stephen Webb, If the Universe Is Teeming with Aliens…Where Is Everybody? (New York: Copernicus Books, 2002), 37.
- Paul Raeburn, “Venus, the Unexplored Planet Right Next Door,” Discover (June 2012): 62–64.
- Hugh Ross, The Creator and the Cosmos, 3rd ed. (Colorado Springs: NavPress, 2001), 184–85.
- Stuart Ross Taylor, Destiny or Chance (Cambridge: Cambridge University Press, 2000), 118.
- Ibid., 123–26.
- Fazale Rana and Hugh Ross, Origins of Life (Colorado Springs: Navpress, 2004), 190–95.
- Gregory Mone, “Frozen. Irradiated. Desolate. Alive?” Discover (November 2012): 30–37.
- Ibid., 32.
- Scientist Britney Schmidt is pursuing the idea that the cracking and refreezing that occurs in Europa’s ice shell could allow mixing between surface and subsurface water. This would permit certain surface chemicals (SO2, peroxide) to reach the water layer where they could then drive life chemistry; see Mone, “Frozen. Irradiated. Desolate. Alive?”, 30–37. However, there are a number of reasons for concluding that Europa could house only a trivial amount of microscopic life, at best, but certainly not advanced life. These include: (1) the enormous pressure of water under miles of ice; (2) all water freezing solid at times due to episodic tidal heating; (3) extremely salty water; and (4) no light and little chemical energy available for metabolism; see Guillermo Gonzalez and Jay W. Richards, The Privileged Planet (Washington, DC: Regnery Publishing, 2004), 88–90. See also Rana and Ross, Origins of Life, 197–201; Peter Ward, Life As We Do Not Know It (London: Penguin Books, 2005), 197–214.
- Peter D. Ward and Donald Brownlee, Rare Earth (New York: Copernicus Books, 2000), 15–21; Gonzalez and Richards, The Privileged Planet, 127–36.
- Gonzalez and Richards, The Privileged Planet, 132.
- Sam Flamsteed, “Impossible Planets,” Discover, September 1997, 78–83; Tim Folger, “NASA’s Inspiring, Enlightening, and Successful Search for New Earths,” Discover, May 2011, https://discovermagazine.com/2011/may/25-inspiring-enlightening-story-nasas-search-new-earths#.UcDMxtiE6E9.
- Hugh Ross, Why the Universe is the Way it is (Grand Rapids, MI: Baker Books, 2008), 62–63.
- Ibid., 180–81.
- Ward and Brownlee, Rare Earth, 205–206.
- Taylor, Destiny or Chance, 142–43.
- Ross, The Creator and the Cosmos, 184–85.





