Ultraviolet Light Illuminates More Problems for Chemical Evolution
When my wife and I visited Australia, our hosts warned us repeatedly to use sunscreen. The thinning ozone layer near the South Pole intensifies the damaging effects of ultraviolet (UV) radiation in Australia. And according to a new study, it appears that UV radiation is also dealing damage to chemical evolutionary hypotheses.1
Origin-of-life researchers have miscalculated the importance of UV radiation for evolutionary models, according to recent work by researchers from the Harvard-Smithsonian Center for Astrophysics (CfA). The CfA scientists modeled the sun’s UV radiation on early Earth and concluded that UV light might not have been an effective energy source to power prebiotic chemical reactions.
How Does UV Radiation Affect the Origin of Life?
Many origin-of-life investigators consider UV radiation one of the most significant energy sources for prebiotic chemistry on early Earth. Planetary scientists estimate that UV radiation from the early sun would have provided three orders of magnitude more energy on Earth than other energy sources.
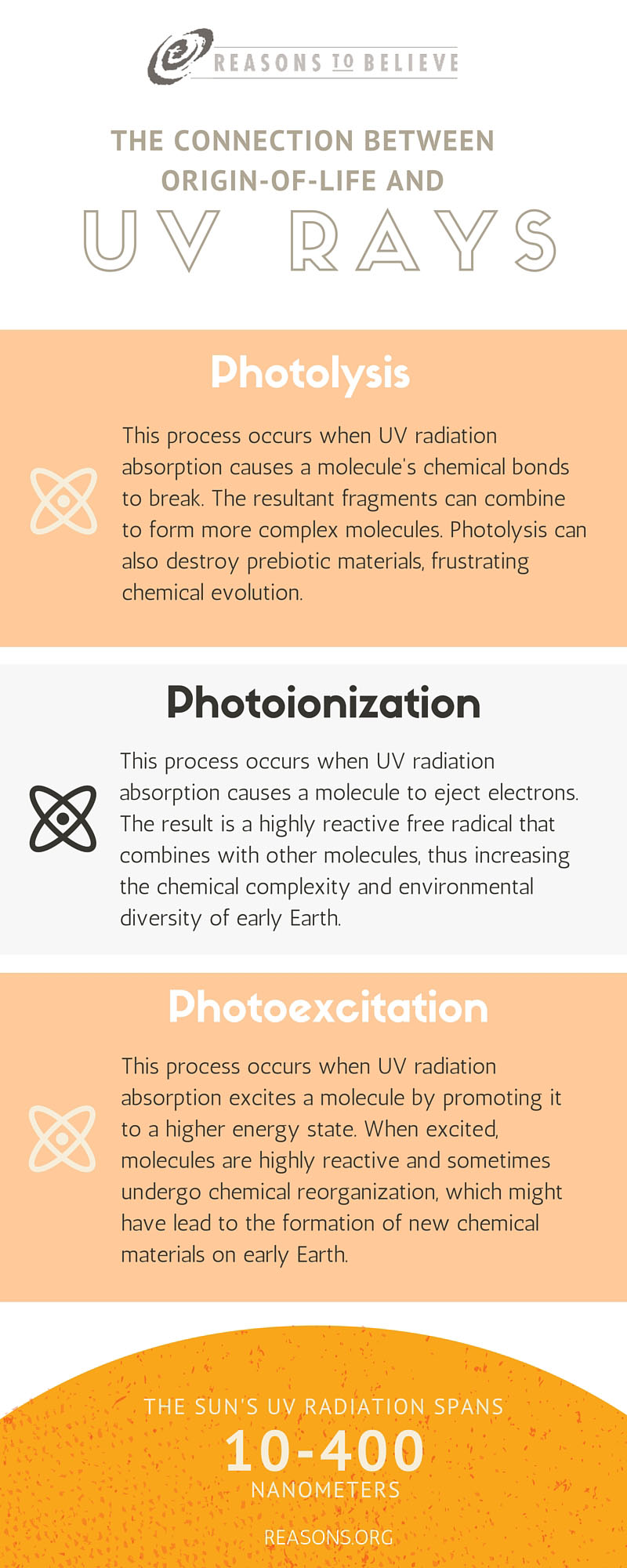
When UV radiation interacts with molecules, it can trigger three different types of chemical processes: (1) photolysis, (2) photoionization, and (3) photoexcitation. Each process would have been important for prebiotic reactions occurring in early Earth’s atmosphere and oceans.
Given its ubiquity on early Earth and its capacity to catalyze chemical reactions, UV radiation figures prominently in many prebiotic chemistry experiments. Typically, researchers will deliver UV radiation to laboratory reactions using a UV lamp, such as commonly used mercury lamps.
However, the concern is that this radiation consists of a single wavelength. (Mercury lamps emit 184 and 254 nanometers, nm.) Solar UV radiation is broadband, spanning wavelengths from 10 to 400 nm. (Photolysis requires between 120 and 1,200 nm. Photoionization requires even shorter—thus more energetic—wavelengths.) Another research concern is that the intensity of the energy from UV lamps might be too high. (Mercury lamps are one to two orders of magnitude more intense than solar radiation.)
These differences between lab conditions and real-world scenarios could have skewed scientists’ understanding of UV radiation’s impact on prebiotic chemistry. This issue prompted the CfA scientists to examine early Earth’s UV radiation environment and compare it to the UV radiation typically used in laboratory prebiotic simulations. In doing so, they hoped to assess the UV radiation’s real contribution to early Earth’s prebiotic reactions and to determine whether the use of UV radiation in laboratory simulations has been realistic.
UV Radiation’s ActualContribution to Early Earth
To investigate these questions, the CfA researchers modeled the UV emission spectrum of the sun at 3.9 billion years ago and then examined how atmospheric and oceanic water attenuated the radiation. The team discovered that atmospheric water shielded early Earth from UV radiation with wavelengths shorter than 173 nm and that liquid water in the early oceans shielded wavelengths shorter than 210 nm. Thus, the effects of both atmospheric and oceanic water would have frustrated a number of potential prebiotic reactions that would have relied on photolysis and photoionization.
Based on this finding, the CfA researchers are cautioning origin-of-life investigators to avoid using fluorine and ArF excimer lasers (which emit light at 158 and 193 nm) or mercury lamps (which emit at 184 and 254 nm) in prebiotic simulation experiments. The inevitable corollary of this study is that any conclusions derived from prebiotic simulation studies using these wavelengths of light are irrelevant to origin-of-life scenarios.
The CfA scientists also discovered that UV absorption by carbon dioxide in early Earth’s atmosphere would have shielded methane and hydrogen cyanide from photolysis. This is a mixed bag of results for origin-of-life researchers. Methane and hydrogen cyanide are key components in many prebiotic reactions. Many researchers believe that photolysis would have destroyed these materials. So, while carbon dioxide would have afforded methane and hydrogen cyanide protection from UV radiation, it also would have prevented photolytic processes from initiating important chemical reactions in which methane and hydrogen cyanide take part.
In effect, the CfA scientists have demonstrated that although it still could have contributed to some prebiotic reactions on early Earth, UV radiation may not be as effective an energy source as previously thought.
UV Radiation in the Laboratory
The CfA scientists analyzed recent high-profile prebiotic studies that employed UV radiation as a critical part of the experimental design. One such study described a newly discovered chemical route that yields the activated ribonucleotides composed of cytosine and uracil (two of the four components needed to build RNA).2 This work has been heralded as important validation of the RNA world hypothesis.3 The researchers who discovered this reaction used UV radiation to trigger the conversion of the cytosine ribonucleotide to the uracil ribonucleotide. They also used UV radiation in the last step of the synthesis to eliminate unwanted side products, thus increasing the reaction’s yield.
In a previous article, I criticized the researchers’ selective use of UV radiation and how they irradiated the reaction during the last step only. On primordial Earth, each step of the reaction would have been exposed to UV radiation. Continuous exposure would have indiscriminately destroyed many of the reactants and products in the earlier steps of the chemical route and shut down the chemical reaction. Not a good scenario for evolutionary origin-of-life models.
The CfA scientists have now pointed out that origin-of-life researchers used a mercury lamp that emitted only a single wavelength of light (254 nm). If that team had employed a broadband source, it is unlikely that the purification of the desired ribonucleotide products would have resulted. Why? It’s because across the UV spectrum, the ribonucleotides and the undesired side products differentially absorb radiation at various wavelengths. In other words, at some wavelengths the side products are more stable to the UV radiation than the ribonucleotides and vice versa. In fact, the researchers showed that when a broadband UV source is used, the UV radiation enhances the yield of the side products, not the desired ribonucleotides.
For the origin of life to have occurred via chemical evolution, an efficient energy source must be identified. Most origin-of-life investigators assume that solar UV radiation would have provided that energy. Thus, they routinely employ UV radiation in prebiotic simulation studies. Yet as the CfA scientists have shown, early Earth’s water and carbon dioxide would have frustrated a number of critical prebiotic reactions. Plus, the use of single-wavelength UV sources in laboratory simulation studies is unrealistic and raises questions about relevancy. The CfA researchers have identified even more problems for naturalistic explanations of life’s genesis, making it less likely that chemical evolution generated life.
Endnotes
- Sukrit Ranjan and Dimitar D. Sasselov, “Influence of the UV Environment on the Synthesis of Prebiotic Molecules,” Astrobiology 16 (January 2016): 68–88, doi:10.1089/ast.2015.1359.
- Matthew Powner, Béatrice Gerland, and John Sutherland, “Synthesis of Activated Pyrimidine Ribonucleotides in Prebiotically Plausible Conditions,” Nature 459 (May 2009): 239–42, doi:10.1038/nature08013.
- See Fazale Rana, “Rescuing the RNA World?, Part 1 (of 2),” Today’s New Reason to Believe (blog), Reasons to Believe, August 13, 2009, https://www.reasons.org/articles/rescuing-the-rna-world-part-1-of-2; Fazale Rana, “Rescuing the RNA World?, Part 2 (of 2),” Today’s New Reason to Believe(blog), Reasons to Believe, August 20, 2009, https://www.reasons.org/articles/rescuing-the-rna-world-part-2-of-2.